Water is essential for life, and its chemical identity is vital to understanding its role in our world.
The scientific name for water is H2O, signifying that each molecule is made up of two hydrogen atoms bonded to one oxygen atom. This simple yet powerful formula underpins countless processes that sustain living organisms, from hydration to cellular functions.
The unique properties of H2O arise from its molecular structure, where the arrangement of atoms creates a polar molecule. This polarity allows water to act as a solvent, enabling it to dissolve various substances crucial for life.
The presence of water on Earth is immense, with oceans covering a significant portion of the planet. For more insights on water’s importance, readers can explore articles that cover various aspects of this life-sustaining compound.
In addition to its physical and chemical characteristics, water is intricately linked to weather patterns and climate.
Meteorologists study its movements, from evaporation to precipitation, to understand weather systems better. Through water’s journey, its role as a key player in environmental and weather phenomena becomes increasingly clear. The interconnectedness of water with every aspect of life and the environment makes it a subject worth exploring further.
Chemical and Physical Properties of Water

Water is essential to life and has unique properties that make it vital for many natural processes.
Its molecular structure and the way it interacts with itself and other substances contribute to its distinctive characteristics.
Molecular Composition and Chemical Formula
Water, known scientifically as H2O, consists of two hydrogen atoms bonded to one oxygen atom. This simple molecular composition plays a key role in defining its properties.
The hydrogen bonds formed between water molecules allow for unique interactions, making water an effective solvent, which means it can dissolve many substances. This quality is crucial for biochemical reactions in living organisms.
Additionally, the polar nature of water molecules helps them to attract and hold ions, supporting many processes essential for life.
Physical States and Transformation
Water exists in three physical states: solid (ice), liquid (water), and gas (water vapor). Its freezing point is 0°C (32°F), while it boils at 100°C (212°F) at standard atmospheric pressure.
Water can easily transition between these states through processes such as melting, freezing, evaporation, and condensation. For instance, when temperature increases, ice melts into water. Conversely, when water vapor cools, it can condense back into liquid water.
This ability to change states is key in weather patterns and the water cycle, impacting climate and ecosystems.
Characteristics Unique to Water
Water has several unique characteristics that make it essential.
Its high specific heat capacity allows it to absorb a lot of heat without a significant rise in temperature, which helps regulate climate. Water also has high surface tension due to the cohesive forces between its molecules.
This property allows small objects to float on its surface and is essential in processes like water transport in plants. Density is another important characteristic; ice is less dense than liquid water, which is why ice floats. These unique traits all contribute to water’s crucial role in supporting life on Earth.
Water Cycle and Its Global Effects
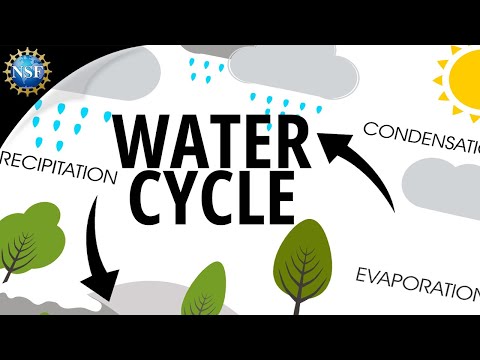
The water cycle is a complex system that involves the continuous movement of water through various phases. It plays a vital role in ecosystems, climate, and human activities. Understanding these interactions helps to highlight the importance of water conservation and management.
Hydrological Phases and Processes
The water cycle consists of several key phases: evaporation, condensation, precipitation, transpiration, and runoff.
Water evaporates from bodies like oceans and lakes, turning into vapor. This vapor then cools and condenses to form clouds. Eventually, it falls back to Earth as precipitation in forms such as rain or snow.
Aquifers are underground layers that store fresh water, playing a crucial role in supplying drinking water. The cycle also includes runoff, which moves water across surfaces, affecting rivers and lakes. This continual movement shapes weather patterns and influences climate conditions.
Water’s Role in Ecosystems and Climate
Water serves as a fundamental component of ecosystems. It is essential for plant growth through processes like transpiration, where plants release water vapor.
This not only supports agriculture but also helps regulate local climates.
Moreover, acid rain can harm aquatic ecosystems and soil health. Contaminated runoff can damage freshwater sources and aquatic life. Understanding these impacts is crucial for maintaining ecological balance and supporting diverse habitats, from wetlands to forests.
Human Interaction and Usage
Humans interact with the water cycle in various ways.
Activities like agriculture heavily rely on fresh water for irrigation.
Desalination processes convert seawater to fresh water, providing solutions in water-scarce regions.
Industrial processes also demand significant water resources, which can lead to pollution.
Aqueducts transport water for cities and farmland.
Practicing conservation helps ensure sustainable water usage and protects the hydrosphere.
Sustainable practices reduce waste and maintain water quality, benefiting both people and the environment.
