Heat is a form of energy that plays a vital role in our understanding of physics.
The laws of thermodynamics explain how heat is transferred between objects of different temperatures and outline the fundamental principles governing energy interactions. These laws establish that heat naturally flows from hot to cold, signifying a key aspect of energy exchange in our universe.
Temperature is not just a number; it reflects the kinetic energy of molecules in a substance.
When heat moves from an area of high temperature to one of lower temperature, it results in various phenomena, from weather changes to the functioning of machines. Understanding these principles is crucial for grasping how energy works in everyday life and in complex systems.
Thermodynamics extends beyond simple definitions, as it encompasses the entire framework of energy exchanges that drive natural processes.
By exploring these laws, readers can gain deeper insights into not only physics but also how heat impacts everything from climate patterns to technological innovations. This knowledge is not just academic; it has practical applications that affect daily life and the environment.
Fundamentals of Heat in Physics

Heat is a form of energy that plays a crucial role in physics, particularly in thermodynamics. Understanding how heat interacts with temperature and energy transfer is essential in various fields, from meteorology to engineering.
This section covers key concepts like temperature, heat transfer methods, and thermodynamic processes.
Understanding Temperature and Heat
Temperature is a measure of the average kinetic energy of particles in a substance. It determines how hot or cold an object is and is often measured in Celsius, Fahrenheit, or Kelvin.
Heat, on the other hand, is the energy that flows from one body to another due to a temperature difference.
Thermal equilibrium occurs when two objects at different temperatures come into contact and exchange energy until they reach the same temperature.
The zeroth law of thermodynamics states that if two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other. This principle is foundational for understanding thermal interactions between bodies and helps in calculating temperature changes and energy transfer.
Forms of Heat Transfer
Heat transfer occurs through three primary methods: conduction, convection, and radiation.
- Conduction happens when heat transfer occurs between materials in direct contact. The heat moves through collisions between particles.
- Convection involves the movement of fluids (liquids or gases) carrying heat with them. Hot fluid rises while cooler fluid sinks, creating a cycle.
- Radiation is the transfer of heat through electromagnetic waves, allowing energy to travel through a vacuum.
Each method plays a significant role in everyday phenomena. For instance, the warmth felt from sunlight is a classic example of heat transfer via radiation.
Understanding Thermodynamic Processes
Thermodynamic processes describe the changes in temperature, pressure, and volume in a system. They can be path dependent or involve state functions.
Processes can be classified into several types, including isothermal (constant temperature), adiabatic (no heat exchange), and isobaric (constant pressure).
A phase transition occurs when a substance changes its state, such as from solid to liquid.
Specific heat is the amount of heat required to change the temperature of a unit mass of a substance by one degree. Understanding these concepts is critical for analyzing heat engines and other systems that rely on heat transfer and transformation. For more on temperature dynamics, see articles focused on temperature.
Thermodynamic Laws and Heat Engines
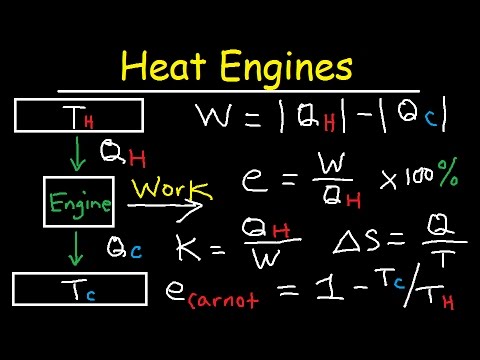
Understanding the connection between thermodynamic laws and heat engines is crucial in the study of energy transformation.
These laws describe how energy is conserved and how it flows within systems, impacting the efficiency of heat engines used in various applications.
Principles of the Laws of Thermodynamics
The laws of thermodynamics outline the principles governing energy transfer and work.
The First Law of Thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed. It can only change forms, for instance, from thermal energy to mechanical energy in a heat engine.
The Second Law of Thermodynamics introduces the concept of entropy, which measures the disorder within a system.
It asserts that in any energy exchange, if no energy enters or leaves, the potential energy will always increase, leading to less usable energy. This law explains why no heat engine can be 100% efficient, as some energy is always lost as waste heat.
The Mechanics of Heat Engines
A heat engine converts heat energy into mechanical work through a cyclic process.
It typically involves a hot reservoir (the source of heat) and a cold reservoir (where waste heat is released).
For example, a steam engine operates by heating water in a boiler until it turns to steam. This steam then expands and pushes against a piston to perform mechanical work.
Heat engines rely on the principles of thermodynamics to function effectively.
Heat sinks are also critical in this process, as they absorb excess heat, allowing engines to maintain optimal temperatures.
The efficiency of heat engines is significantly influenced by the aforementioned laws, which dictate how well energy is conserved and utilized.
