Stratospheric ozone plays a crucial role in protecting life on Earth by filtering harmful ultraviolet (UV) radiation from the sun. Despite its importance, this protective layer is being weakened significantly, leading to various environmental issues.
Ozone depletion occurs primarily due to human-made chemicals known as ozone-depleting substances (ODS), such as chlorofluorocarbons (CFCs), which release chlorine and bromine into the atmosphere.
The thinning of the ozone layer is most pronounced in the polar regions, especially over Antarctica.
As ODS continue to break down ozone molecules, the balance between ozone production and destruction is disrupted, resulting in increased UV radiation reaching the surface. This rise in UV exposure can have serious consequences for human health, ecosystems, and wildlife.
Understanding the causes behind stratospheric ozone destruction is essential for addressing this environmental crisis. By learning more about the role of ODS and the impact of ozone layer depletion, individuals can become informed advocates for the protection of our atmosphere and the preservation of life on Earth.
Mechanisms of Ozone Layer Depletion
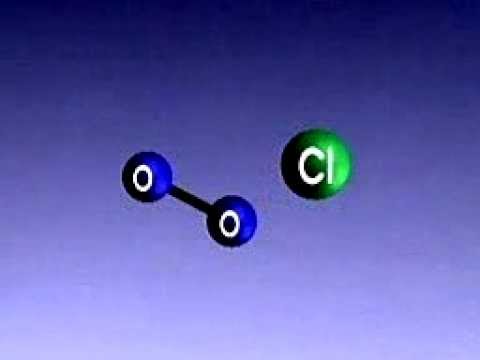
Stratospheric ozone depletion occurs due to various chemical processes, primarily involving human-made substances. Understanding these mechanisms is essential to grasp the extent of the problem and its impacts on the environment.
Chemical Reactions Involving Chlorine and Bromine
Chlorine and bromine play critical roles in ozone layer depletion.
When ozone-depleting substances like chlorofluorocarbons (CFCs) and halons are released into the atmosphere, they eventually rise to the stratosphere. Here, they undergo photodissociation under the influence of solar radiation.
This process releases chlorine and bromine atoms, which can react with ozone (O₃) molecules. One chlorine atom can destroy up to 100,000 ozone molecules through a series of chemical reactions. The breakdown of ozone leads to the formation of more oxygen molecules (O₂), depleting the ozone layer and contributing to the formation of the ozone hole.
Role of Human-Made Substances
Human-made substances significantly impact the ozone layer.
CFCs and hydrochlorofluorocarbons (HCFCs), commonly used in refrigeration, air conditioning, and aerosol products, are major culprits. When these substances are released, they persist in the atmosphere for many years.
Other ozone-depleting substances include halocarbons, carbon tetrachloride, and methyl bromide. These compounds break down ozone through chemical reactions that release harmful chlorine and bromine. Although the production of many of these substances has been banned or restricted due to international agreements like the Montreal Protocol, their lingering effects still pose challenges for ozone recovery.
Natural Factors Influencing Ozone Levels
Natural factors also contribute to stratospheric ozone depletion.
Volcanic activity can release gases that may influence ozone levels. In addition, polar stratospheric clouds (PSCs) form under cold conditions in the stratosphere, especially during winter in polar regions.
These clouds provide a surface for chemical reactions to occur, enhancing the destruction of ozone. As sunlight returns in spring, reactions on PSCs release ozone-depleting chlorine and bromine, leading to significant ozone loss, especially over Antarctica, creating the well-known ozone hole.
Regulatory and Protective Measures

Efforts to combat stratospheric ozone depletion focus on international cooperation, technological advances, and ongoing monitoring. These measures aim to reduce harmful emissions and promote the recovery of ozone concentrations.
International Agreements and Protocols
Major international agreements play a crucial role in protecting the ozone layer. The Montreal Protocol, established in 1987, is a landmark treaty that successfully reduced the production and consumption of ozone-depleting substances (ODS). This agreement phased out chemicals like chlorofluorocarbons (CFCs) and halons.
The protocol has been amended several times to address newer substances, including hydrofluorocarbons (HFCs), which, while initially deemed ozone-friendly, have high global warming potential. The amendments aim to ensure that all countries, regardless of development status, can contribute to ozone recovery.
Advances in Alternative Technologies
Technological innovations have paved the way for using ozone-friendly alternatives in various industries.
In refrigeration and air conditioning, substitutes like hydrocarbon-based refrigerants and natural refrigerants, such as CO2 and ammonia, offer effective options that do not deplete the ozone layer.
Companies are increasingly investing in these safe alternatives, reducing reliance on harmful chemicals. Additionally, advancements in energy efficiency help mitigate greenhouse gas emissions. The growth of eco-friendly technologies not only supports environmental protection but also provides opportunities for economic growth in green industries.
Monitoring and Researching Ozone Recovery
To ensure the effectiveness of regulations, ongoing environmental monitoring and research are critical.
Agencies like the U.S. Environmental Protection Agency (EPA) and international organizations track ozone concentrations using satellite data and ground-based measurements.
Scientific research also focuses on understanding the complexities of ozone layer dynamics and the impacts of human activity.
This growing body of knowledge is essential for assessing the success of international agreements and guiding future policy decisions.
With continued monitoring, scientists can evaluate the strides made towards ozone recovery and adjust strategies as needed to protect this vital component of Earth’s atmosphere.

